Nanotechnology Abounds at the Capstone
By Chris Bryant and Mary Wymer
Photos by Chip Cooper, Zach Riggins and Laura Shill
Think, for a second, about the size of a red blood cell. Now, imagine anything that’s about 7,000 times smaller. That would be a nanometer. It’s also representative of the world in which a host of University of Alabama researchers are involved.
From efforts to embed the tiniest of treatments within individual cancer cells, to harnessing the power of a sophisticated instrument capable of determining both the positions and types of atoms within materials, UA researchers are poised to develop big things by thinking small.
A BIG LEAP FORWARD
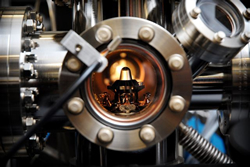
UA’s latest equipment acquisition in the research of the very small is called a Local Electron Atom Probe, know as a LEAP. Acquired from Imago Scientific Instruments, the highly sophisticated microscope permits UA researchers to determine the positions and types of atom in various materials and view the structure in 3-D.
“This is significant,” said Dr. Mike Bersch, director of UA’s Central Analytical Facility, “because we know – at both the macro- and nano-scales – a material’s properties, such as strength or conductivity, depend upon which atoms are where,” he says.
The University of Alabama is the first academic institution in the Southeast, and only the fourth in the nation, to obtain Imago’s most advanced atom probe, the new LEAP Si, according to a company representative.
“Through the LEAP’s analysis, researchers can better design materials with tailored properties for such applications as transistors used in cell phones, hard drives for computers, or high-strength, low-weight steels used for fuel efficient automobiles,” says Dr. Gregory Thompson, assistant professor of metallurgical and materials engineering at UA.
Thompson was the lead principal investigator on a $500,000 National Science Foundation grant won by him and his colleagues for a laser upgrade for the instrument, expanding its analytical capabilities.
UA’s instrument is ideal, says Bersch, for studying semiconductors, the base materials used in manufacturing computer chips and other electronic devices. Additionally, the LEAP will strengthen Alabama’s existing industries, particularly the development of new light-weight, high-strength materials for automobiles. UA researchers are already collaborating with Nucor Steel, Western Digital, Plasma Processes Inc. and Phifer Inc. in this capacity.
“We’ve built a major research instrumentation center related to nanotechnology,” says Bersch.
NANO NOT NEW
Dr. David Nikles knows some of the big things that can be accomplished by thinking small. For 17 years, this UA chemist has worked with the University’s Center for Materials for Information Technology. Known as MINT, the Center focuses on developing new materials to advance data storage.

Storing information on magnetic media – computer hard drives, CDs, audio and video tape is, inherently, a nanoscience. “If people want to point to an example of a nanotechnology that actually is in the marketplace, it’s magnetic hard drives and magnetic tape drive systems,” says Nikles.
Magnetic media, at its core, contain thin films with tiny particles acting as magnets. Computers and other devices arrange these particles so their configurations can later be “read” back as information, be it sound, data or images. For storage capacity to increase, these particles must get smaller. Easily said and not so easily achieved, yet the industry repeatedly accomplishes just that.
“Our mission,” Nikles says, “has been to learn how to make those particles very small.” Presently, MINT can make them as small as 3 nanometers while keeping them thermally stable so they remain reliable.
This, in combination with many other developments, continues to revolutionize electronics on what sometimes seems an hourly basis.
“When I started here in 1990, the highest density hard drive I could get held 80 megabytes,” Nikles recalls. “Now, you can get a terabyte hard drive.” That’s more than a thousand fold improvement in storage capacity. “Same sized disk, same price. A lot of this is materials science – improved magnetic recording materials, improved magnetic materials for heads (which read the stored data).”
There have been other contributors to the overall improvements, Nikles points out, particularly advances in signal processing.

More than 30 faculty from seven academic programs comprise MINT. This research program was the first in the South to be designated as a National Science Foundation Materials Research and Science and Engineering Center when it achieved that highly sought designation in 1994. MINT has won three such awards from NSF. The latest award, $6 million, is to be distributed annually through 2008. MINT’s corporate sponsors include IBM, Seagate, Western Digital, Maxell and Fujitsu.
NANO BATTLES CANCER
Today, Nikles is applying knowledge gained from decades of information storage research to the fight against cancer. Working with scientists at the University of Alabama at Birmingham’s Gene Therapy Center, and other UA colleagues and students, Nikles is developing a way to piggyback nanoparticles onto a genetically engineered human virus. The virus and its miniscule sidekick would then be injected into the bloodstream.
“We have a team of chemists and chemical engineers that are synthesizing the magnetic particles and designing the linkers that bind to the particle and also bind to the (virus) surface,” Nikles said. “You could imagine that the magnetic particle is tethered to the virus surface with covalent bonds (a chemical bond formed between atoms when they share electrons) that are strong enough that the particles get pulled along as the virus travels to find the cancer cells.”
The group takes a multi-pronged approach. In one, they seek to use nanoparticles of rust as a contrast enhancement agent, improving the likelihood of MRI’s revealing cancer cells that have already metastasized, or spread, from the primary tumor to others parts of the body.
“You could think about it as decorating the cancer cells,” Nikles said. “If you can have a much better sensitivity for magnetic resonance imaging, you could think about going after metastasized cancer cells.”
In another approach, the scientists are looking at ways to use nanoparticles in hyperthermia therapy, the damaging and killing of cancer cells by heating surrounding tissue. Embedding the particles within the cancerous cells would provide a means to use an AC field to generate enough heat to damage or kill the cells, Nikles said.
In 2006, Nikles co-authored a paper describing the work that published in the online edition of Nano Letters. “In that paper, we were demonstrating that if you put a well defined 2 nanometer gold particle on a virus, the gold particles do not interfere with the ability of the virus to find the cancer cells and to enter the cells.”
Thirdly, Nikles and his colleagues work closely with Dr. Christopher Brazel, a chemical and biological engineering faculty member. In this scenario, researchers develop a nano-sized polymer that would contain an existing cancer drug. The polymer, with the drug enclosed, would also be attached to the adenovirus genetically altered by UAB researchers.
“Instead of the heating killing the cancer cells, the heating heats the polymer composite to drive it above its transition temperature so that it releases the drugs.”
STRIVING TO REDUCE CHEMO’S SIDE EFFECTS

The overall goal of the team, which includes physicists, material scientists, biologists, geneticists and physicians, is to maximize treatment possibilities while minimizing side effects, says Dr. Christopher Brazel, an associate professor of chemical and biological engineering. Successfully delivering nanoparticle chemotherapy to individual cells and activating those particles involves understanding the properties of heat through magnetism and how that heat affects the surrounding healthy tissues, Brazel says.
“A lot of these concepts are years away,” Nikles said. “The National Cancer Institute has targeted the year 2015 for curing cancer. They see nanoscience as a tool.”
While such cancer cures are conceptual, and concerns, such as the potential toxicity of any nanoparticles used, are still to be resolved, Nikles said the group has made some key strides.
“We’ve come up with procedures where we can control the particle diameter and the particle composition,” he said. “These particles are so small and the cells so large, relative to the particles, that they can penetrate subcellular structures. I believe that the particle size and its surface chemistry will affect where within the cells the particles might go.”
Therefore, being able to exactly replicate the size of the particles is a key, Nikles said. “At the nanoscale – and this is a fundamental lesson of nanoscience – the size of the particle strongly affects its physical properties. We know about the physical properties of the bulk material, where the size of the particles is huge, but when you get down to the nanometer scale, the physical properties vary with size in a way that’s not well understood. That’s the science of nanoscience – understanding how they vary.”
NEXT GENERATION OF NANO RESEARCHERS

In addition to performing world-class research, Nikles and his colleagues, like Dr. Martin Bakker, associate professor of chemistry, are also interested in teaching tomorrow’s scientists the world of nano.
Bakker organizes MINT’s Research Experience for Undergraduates program, or REU. Supported by the National Science Foundation, as is a similar program hosted by UA’s chemistry department, the program provides a variety of research experiences to top undergraduates nationwide in an attempt to spark more interest in science among students.
“We give strong preference to people from small schools where they don’t have the same opportunity to do research as they would at a UA,” Bakker says. The program also seeks to increase cultural and gender diversity in the field by involving groups typically underrepresented in the sciences.
Students apply for the program and, if accepted, spend multiple weeks working on individual and group research projects while being mentored by UA faculty and graduate students. It’s here that some of the students are, for the first time, exposed to nanoscience, Bakker says.
“Nanoscience is something that still is not really penetrating the undergraduate curriculum,” Bakker says. Although that’s beginning to slowly change on some campuses, in many fields it’s only through research that undergraduate students are exposed to nanotechnology, he says. This can also help eliminate some of the unease some feel regarding nanoscience.
“When you talk about nano, a lot of the general public, if they know about it, it’s intimidating. ‘This is cutting edge science, it must be difficult,’” is the approach many take, Bakker says. “Sure, some of the details are challenging, but a lot of this stuff, if you think about how it works, you can see, ‘yeah, I can do that.’”

Nikles and Thompson have even taken the approach a step further, creating, four years ago, an internship program for high school students that focuses primarily on nanoscience.
NANO PROVIDES POWER SURGE
Bakker and his colleagues also seek to overcome some of the shortcomings of today’s batteries through the use of nanoscience. The UA chemist holds up a standard AA battery. “There is probably enough energy stored in this battery to start your car. You might only be able to do it once. Why is it that this small battery has enough energy, but you use a cell this size?” he asks, while gesturing the approximate length of a car battery. “You are limited in how fast the electricity can come out,” he answers.
“We are looking at ways of improving that by minimizing the distance electricity has to flow.” Through nano- and micro-structured approaches, scientists like Bakker are developing supercapacitors. Such devices have the capability of providing a high surge of electricity within a short time frame as the electrons they contain only have to flow minute distances.
The concept can become clearer, Bakker suggests, if you think of the ease of travel a well designed highway system permits in a large city.
“If all we had were two-lane highways and you had to go across town, it would be dreadfully slow. So, you put in expressways every so often. Then, you put in four-lane highways that take you to an expressway. And, all of a sudden, you can get across Chicago in half an hour. That is really very much the same thing we’re looking at in supercapacitors. With a regular battery, in a sense, you don’t invest in putting in superhighways. It’s all two-lane highways. Yeah, you can get plenty of cars out of it, but it takes time. Whereas, by doing microstructuring and nanostructuring, you have sort of brought in that type of improved highway infrastructure.”
IT’S ALL ABOUT SURFACE AREA
Key to nanomaterials’ role in capacitors, and in many other applications, is the increased surface area they provide, Bakker says. For example, think of a board game’s die, measuring 1 centimeter per side. Combining each of the die’s six sides gives 6 square centimeters of surface area. However, if that same sized die was actually filled with smaller dies, 1 millimeter on a side, it could hold 1,000 such cubes. The surface area of these 1,000 cubes, combined, provides 60 square centimeters of surface area, 10 times the amount of surface area the original 1 centimeter die contained. Apply this principle at the nanometer scale and the increased surface area soon gets mind boggling.
“You might have a football field’s worth of storage area in something the size of a double-A battery,” Bakker suggests. This is significant because energy can be stored on those surface areas.
“The other way batteries work,” Bakker says, “is by storing the charge as chemical energy. There isn’t any reason why you can’t combine those two approaches and build a very high surface area and also build a very good access to all your material that will also store the charge chemically.”
HERE COMES THE SUN
Dr. David Dixon, a computational chemist at UA, is among scientists exploring how nanotechnology could make solar energy more practical.
Of all the alternative energy solutions being explored today, only solar is available in sufficient quantities to meet the world’s future energy needs, says Dixon. “We have to capture the energy in sunlight more efficiently,” he says.
Dixon leads a Nanoscale Interdisciplinary Research Team, sponsored by the National Science Foundation. Along with his UA chemistry colleague Dr. Greg Szulczewski and researchers at Georgia Tech and Case Western, the group, with $1.1 million in NSF funding, is exploring ways to improve titanium dioxide’s ability to convert solar energy to electrical energy.

Titanium dioxide is a naturally occurring mineral that is processed and used as a pigment in a variety of ways, including as a common component in paint. “It’s what makes paint white and is also used in paper,” Dixon says.
This non-toxic, inexpensive pigment naturally absorbs ultraviolet sunlight. The researchers are exploring ways to alter the material so that it absorbs light more efficiently from the visible solar spectrum.
It’s well known among scientists, Szulczewski says, that more of the sunlight can be captured by chemically substituting nitrogen atoms for some of the oxygen atoms within titanium dioxide nanoparticles.
“We are trying to fundamentally understand the physical structures of the doped (or altered) titanium dioxide and where the nitrogen atoms are,” he says. “The idea is to understand why does substituting nitrogen for oxygen give us this change? Could we substitute different atoms than nitrogen that will work even better? How would we go about doing that?”
The researchers take titanium dioxide particles and chemically manipulate them into specific shapes and structures at the nano level, Dixon says. A technique developed by one team member, Georgia Tech’s Dr. James Gole, enables improved control and flexibility in manufacturing the nanoparticles and improving their performance, Dixon says.

Producing nanoparticles isn’t the trickiest part, producing them uniformly is, Szulczewski says. Without such consistency, experiments done to understand the particles properties are not accurate.
“If you don’t have exactly the same size and composition, you are measuring some average properties, and we might miss what specific type of nanoparticle is the most important one.”
Improving titanium dioxide’s ability to convert solar energy to electricity would enable the direct splitting of water into hydrogen and oxygen. Hydrogen generated from this process can be used as a non-polluting fuel for vehicles.
AN ALTERNATIVE TO $1,200 AN OUNCE
Fuel cells, as replacements for gasoline powered engines, hold promise as a means of reducing dependence on foreign oil and reducing pollution. It’s a promise not without obstacles – particularly cost.
University of Alabama researchers are exploring ways to overcome some of these obstacles, and nanotechnology may prove one of the solutions. Hydrogen fuel cells, for example, produce an electrical current capable of powering an engine. These fuel cells require a substance, known as a catalyst, to activate the hydrogen. Platinum, for instance, does a fine job when dispersed as nanometer-sized particles on a support made from carbon powder. However, with a cost typically exceeding $1,200 an ounce, this noble metal contributes to the high cost of a fuel cell. A team of engineers and scientists at UA is developing and testing new types of metal nanoparticles and supports for fuel cell catalysts.
“The ultimate goal of the work is discovery of stable, effective catalysts that substantially reduce or replace the noble metals traditionally required in fuel cell systems,” says Dr. Alan Lane, professor of chemical and biological engineering.
SMART CLOTHES START WITH SMART FIBERS

A rechargeable battery you wear like a vest or protective suits that change colors to signal a contaminant’s presence: these are just two examples Dr. Robin Rogers can imagine for the next generation of smart clothes – made possible through nanotechnology.
UA chemists have taken cellulose – which naturally occurs in the cell walls of trees and other plants – and dissolved it within a new class of environmentally friendly solvents, known as ionic liquids. The researchers then suspend nanoparticles within the solution.
“We have set up a fiber spinning apparatus in the lab,” says Rogers, “and we can pull fibers from the solution that are cellulose, embedded with a functionality. That functionality will be whatever nanoparticle we put in.”
Those fibers could then, theoretically, be used to make clothes that have almost superhero-type traits, including increased strength, or even electrical conductivity.
“I’m very interested in trying to develop advanced textiles such as body armor or a fiber that will be conductive,” says Rogers, the Robert Ramsay Chair of Chemistry at UA. The College of Arts and Sciences faculty member, who led a team that earlier won an Environmental Protection Agency award for cellulose related discoveries, says he’s seeking collaborators to expand upon the developments.
“We’ve taken it to the point where we can control the compositions and the forms, but in order to develop a real product that people want to use, it’s time to collaborate with people who make those products on a regular basis.”
The possibilities, Rogers said, seem almost endless.
“Imagine that you have a nanoparticle that changes color in the presence of a contaminant. Maybe it’s a nerve gas or radioactive material and that you have made a first responder’s suit that’s bright yellow, but, whenever you walk into a hazardous area, it changes color. You would have, essentially, a sensor device.”
Or, how about powering your cell phone, iPod, and laptop with the clothes on your back? “I’ve always liked the idea of developing a battery vest where your personal power device is actually what you are wearing,” Rogers says. Developing possibilities for nanoparticles is, for Rogers, the fun part of the science of the small.
A nanoparticle is a particulate, Rogers reminds. “So, what do you do with it?” he asks. “How do you use it? How do you contain it? How do you spread it? There are a lot of ways that all these things can be done, but our way is that we can encapsulate the nanoparticles in a biorenewable, readily available, very cheap polymer, cellulose, and then use them.”
A GENERAL APPROACH
Bakker says nanoscience developments are, in some ways, like a versatile hand tool. “Yes, you have a spark plug wrench in your toolbox, but most of us also have an adjustable wrench that we can use for the washing machine, to fix the door and work on the car. That’s a little bit of the idea behind nanoscience, as well. It’s a general toolbox of approaches that you can use to take on a bunch of different problems.”
Further Reading